Commissioning is Critical to Validating Containment in the Biosafety Level-3 (BSL-3) Environment

Design standards, biosafety level criteria, and performance requirements are continually evolving to keep pace with the rapidly changing technologies used in Biosafety Level-3 (BSL-3) laboratories. For BSL-3 laboratory mechanical systems, the ventilation and specific design features are vital to containment and protecting workers, the community, and critical research. Commissioning is an essential risk management strategy because it ensures systems are purchased, installed, and perform as intended, validating that containment measures are in place and operating properly. As key stakeholders in the construction and maintenance of BSL-3 laboratories, it is important for health and safety and biosafety professionals to understand the role of commissioning and how system performance impacts containment.
A simple construction fact is that, in the absence of a Quality Assurance (QA) function, systems can operate but fall short of the intended designed performance. A breach of containment in the Biosafety Level-3 laboratories could potentially have a serious adverse impact on worker safety, research integrity, and the community.
The commissioning process provides a QA structure to ensure that the BSL-3 systems are designed, purchased, installed, started, and verified to provide the integrity expected in the critical biocontainment environment. By implementing this QA function, risks are managed in advance of laboratory operations.
Containment in the Biosafety Level-3 Facility (BSL-3)
The Biosafety in Microbiological and Biomedical Laboratories (BMBL), 6th Edition defines two lines of defense that work together to provide containment. Primary containment, which addresses laboratory equipment and work practices; and secondary containment, which focuses on the architectural elements of the laboratory itself, as well as the Mechanical, Electric, and Plumbing (MEP) systems serving the laboratory.
The commissioning process focuses on the secondary containment through physical verification, system testing, trend data analysis, training and maintenance.
Commissioning Process
In general terms, commissioning is defined as a systematic process of review, verification and testing to ensure that equipment and systems perform as intended. The process should ideally span from design through to operation to experience its full value. Early review of the architectural and MEP system design, submittals, and control sequences will ensure the original design intent and identify unwanted deviations. Additionally, these early QA tasks are proven to help with schedule adherence and cost control due to potential changes late in the project.
An essential element of system performance verification is the review of performance data during different operating scenarios via building automation system trend logs. Every operational mode–normal occupied, unoccupied, variable occupancy, emergency and failure–needs to be fully analyzed.
Due to the dynamic nature of building system performance caused by equipment repair and maintenance (control sensor failure and calibration, space use changes and configuration, controls programming, etc.), as-built documentation, staff training, and re-commissioning are critical in maintaining containment in biological laboratories.
Biocontainment Facility Commissioning
Process
Although the commissioning process is the same for any system and space use, for the BSL-3 environment, the level of rigor and impact of failure can vary substantially. Commissioning verification is crucial as assurance that the secondary containment barrier in a BSL-3 laboratory performs, preventing pathogenic microorganisms from leaving the laboratory boundary.
In 2014, the Federal Select Agent Program (FSAP) issued a policy for BSL-3s and animal BSL-3s (ABSL-3) that prior to operation, the verification of design must be performed and documented by an individual with expertise in HVAC systems. The policy also states that documentation provided to FSAP of verification of HVAC design functionality must demonstrate that under exhaust fan or normal power failure conditions, or during normal power start-up, there is no reversal of air which originates within the BSL-3/ABSL-3 laboratory or vivarium room that travels all of the way outside the containment boundary.
Envelope Integrity
A key component in maintaining secondary containment is the integrity of the biocontainment laboratory envelope (walls, ceiling, floor, and doors). The laboratory secondary containment is a system in itself inclusive of both the HVAC system and the architectural elements making up the laboratory envelope. The “tightness” of the space directly impacts the HVAC system capabilities, which have a designed capacity. A “leaky” envelope (boundary) will impact HVAC capacities and the ability to maintain negative pressure.
The Centers for Disease Control and Prevention (CDC) and National Institutes of Health (NIH) require documentation that all laboratory secondary components are installed and completed as designed. The following is a partial list of requirements that will be reviewed for inclusion in the project design phase and verified during construction or prior to testing.
- Laboratory access restrictions
- All laboratory envelope penetrations sealed
- Ventilated airlocks between outside corridor and BSL-3
- Ventilation to be single pass air
- BSL-3 suite to be negatively pressurized to the outside and to surrounding spaces
- All supply and exhaust dampers shall be gas-tight
- Access panels for all mechanical equipment within suite supplied with gas-tight gaskets
- All light fixtures gasketed at point of entry into suite
System
System performance for critical space containment is dependent on equipment quality, equipment redundancy, controls interlocking, and controls timing. Containment results from creating a negative pressure in the space by having greater exhaust air quantities than supply air. In a containment scenario, exhaust air is the “lead” system and supply air tracks the exhaust by a fixed differential to maintain the negative pressure. The two systems are “interlocked” where a change to one system causes a similar reaction from the other. This applies to both system modulation as well as system failure. In the case of system modulation, the programmed offset ensures that as the supply system ramps up or throttles back, pressure is maintained. Upon a complete exhaust system failure, the interlocking program causes the supply system to also shutdown but, in this case, containment is dependent on both the timing of fan and damper closing and the tightness of the seal. Control system selection, sequencing and programming, as well as equipment selection, is critical.
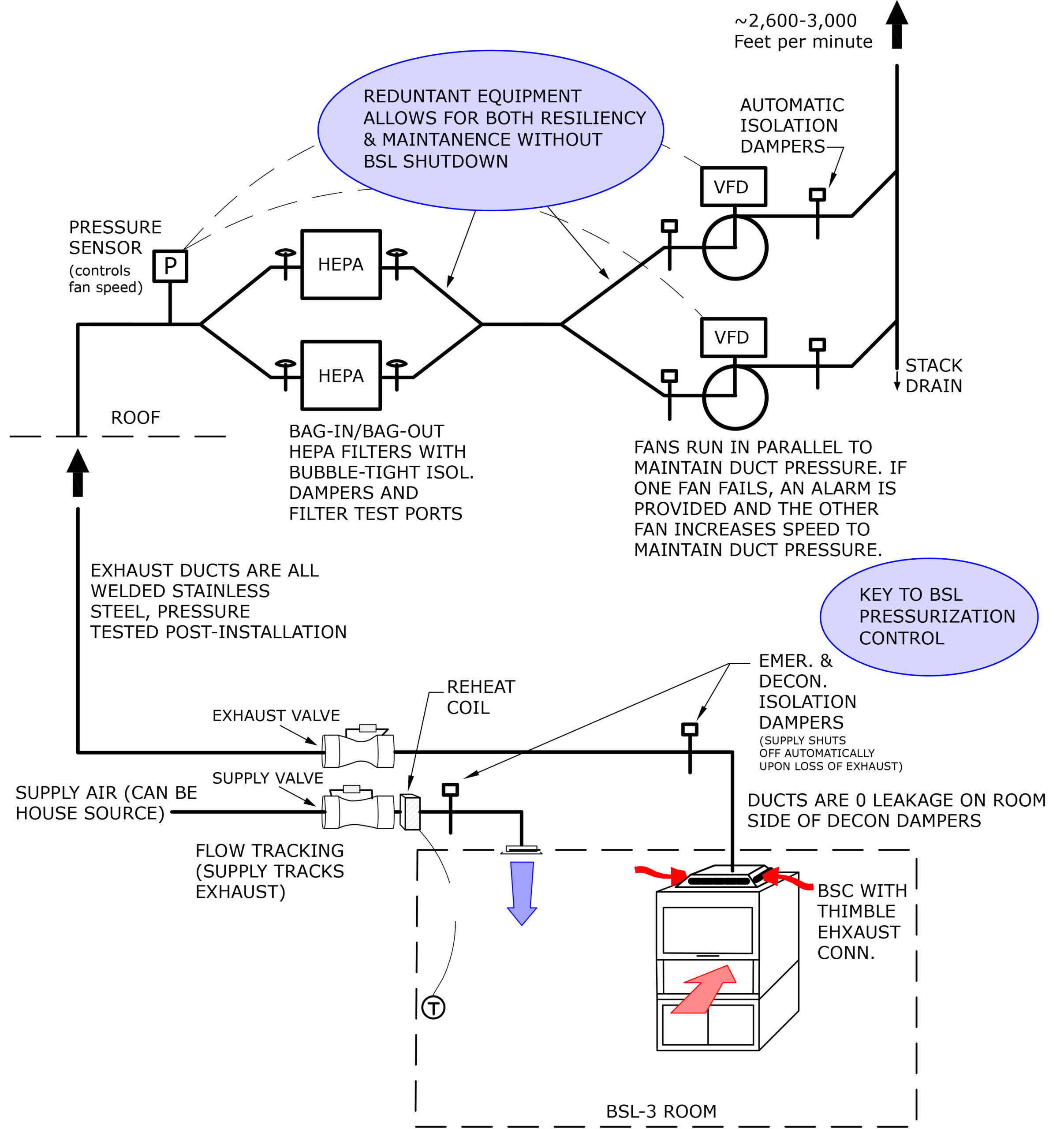
System Testing
Upon completion of construction, which includes controls and system balancing, testing of the secondary containment system will begin. Testing includes all components and control sequences through all modes of operation: occupied, unoccupied, component failure, and emergency power.
Prior to starting formal testing, baseline conditions are established by documenting “as found” or “steady state” operating conditions.
During test procedures, it is important to log the pressure data because that will specifically document the test impact and containment integrity.
Recommended Verification Requirements
The FSAP policy statement lists the minimum BSL-3 verification requirements to be documented upon laboratory completion and annually (these also generally apply to negatively pressurized laboratories as “best practice”).
Upon completion of both physical examination, performance testing, corrective action and final verification, the integrity of the secondary containment barrier is documented. Any incidents resulting from a primary containment breach should stay contained within the laboratory envelope.
However, building systems are dynamic and affected by maintenance, component failures, system changes, related building system influences and research activity changes. Both training and periodic system performance verification must happen to ensure reliability of the containment barriers.
Training
Staff training is critical to ensuring performance over the system life. Training needs to be included in the project commissioning plan with specific attention to who needs to be trained, the training agenda and who the trainer should be. The maintenance staff needs to be fully educated on all facets of the system and ramifications of failures. It is imperative that the user groups be trained on the details and intent of the secondary containment barrier as well as protocol for alarm situations (in addition to specific biosafety laboratory practices and processes).
Re-commissioning
Due to the critical nature of the secondary containment system, it is suggested that re-commissioning should occur on an annual basis or, as recommended in the 2014 FSAP Policy Statement, when there are “major changes to” or “major problems with” the laboratory HVAC system.
The re-commissioning effort will repeat the initial commissioning testing protocol and will include the data trends–demonstrating the continuation of airflow into the laboratory during normal, failure, and emergency operational modes. Ideally, re-commissioning takes place during scheduled maintenance, cleaning or upgrade BSL-3 shutdowns.
Additionally, for a functioning BSL-3 laboratory, it is important to work with stakeholders to determine if the laboratory will be decontaminated with vaporized hydrogen peroxide or a similar space decontaminant prior to launching re-commissioning activities. Alternatively, commissioning personnel will need to follow all requirements (PPE, medical surveillance, etc.) as the laboratory will be considered to be active.
To conclude, commissioning provides the required documentation demonstrating the integrity of the biocontainment laboratory secondary containment barrier. Through the process, all system deficiencies are identified and corrected. Training staff on the importance of the secondary containment barrier as well as how to react in the case of a breach, are key to sustaining continued optimal performance.
Ultimately, implementing the commissioning process will reduce the risk of a pathogen leaving the laboratory boundary and impacting the outside community.
If you’re looking for an expert to verify containment of your BSL-3 space, contact us today. Our commissioning and biosafety professionals are here to help!
Subscribe
to our blog
"*" indicates required fields




